Prepares and protects patients for a stronger start to therapy
The National Immunocompromised Vaccination Program (NIVP), provided by BioScript Solutions®, is designed to simplify access to specialty care by supporting specialists and clinic teams with coordinated, guideline-driven vaccination readiness. Aligned with guidance from the National Advisory Committee on Immunization (NACI) and the Canadian Immunization Guide, this program helps reduce delays to treatment initiation while supporting safe, efficient, and transparent care coordination.
In practice, vaccination pathways can be fragmented and patient-driven, which may lead to:
- missed vaccines,
- incomplete documentation, and
- avoidable delays in care.

Benefits for clinics and patients
- Enhances the patient journey and optimizes health outcomes
- Improves therapy start times and patient readiness
- Diminishes burden to clinicians and patients
About BioScript Solutions and the NIVP program
BioScript Solutions – including BioScript Pharmacy®, Coverdale Clinics®, and AccessLink™ – partners with clinicians to provide comprehensive end-to-end support.
Our experience spans vaccination readiness, patient support, and treatment preparation, helping clinics deliver care more efficiently while maintaining professional and regulatory standards.
Our vaccine services include:
- coordinating patient outreach,
- coordinating of TB and/or viral serology testing,
- collecting vaccination histories,
- scheduling appointments,
- dispensing and administering vaccines, and
- delivering post-injection reports back to the clinic.
This collaborative approach supports transparency, reduces administrative burden, reduces future health care costs, and helps keep care moving forward.
For more information about BioScript, click here.
add
The NIVP supports vaccines recommended for immunocompromised patients in accordance with NACI guidance.
Vaccines supported through the program include:
- hepatitis A and B,
- human papillomavirus (HPV),
- measles,
- mumps and rubella (MMR),
- varicella,
- polio,
- meningococcal,
- pneumococcal,
- respiratory syncytial virus (RSV),
- shingles, and
- tetanus, diptheria, pertussis.
Annual vaccines, such as influenza and COVID-19, are not included. All vaccination decisions remain clinician directed.
add
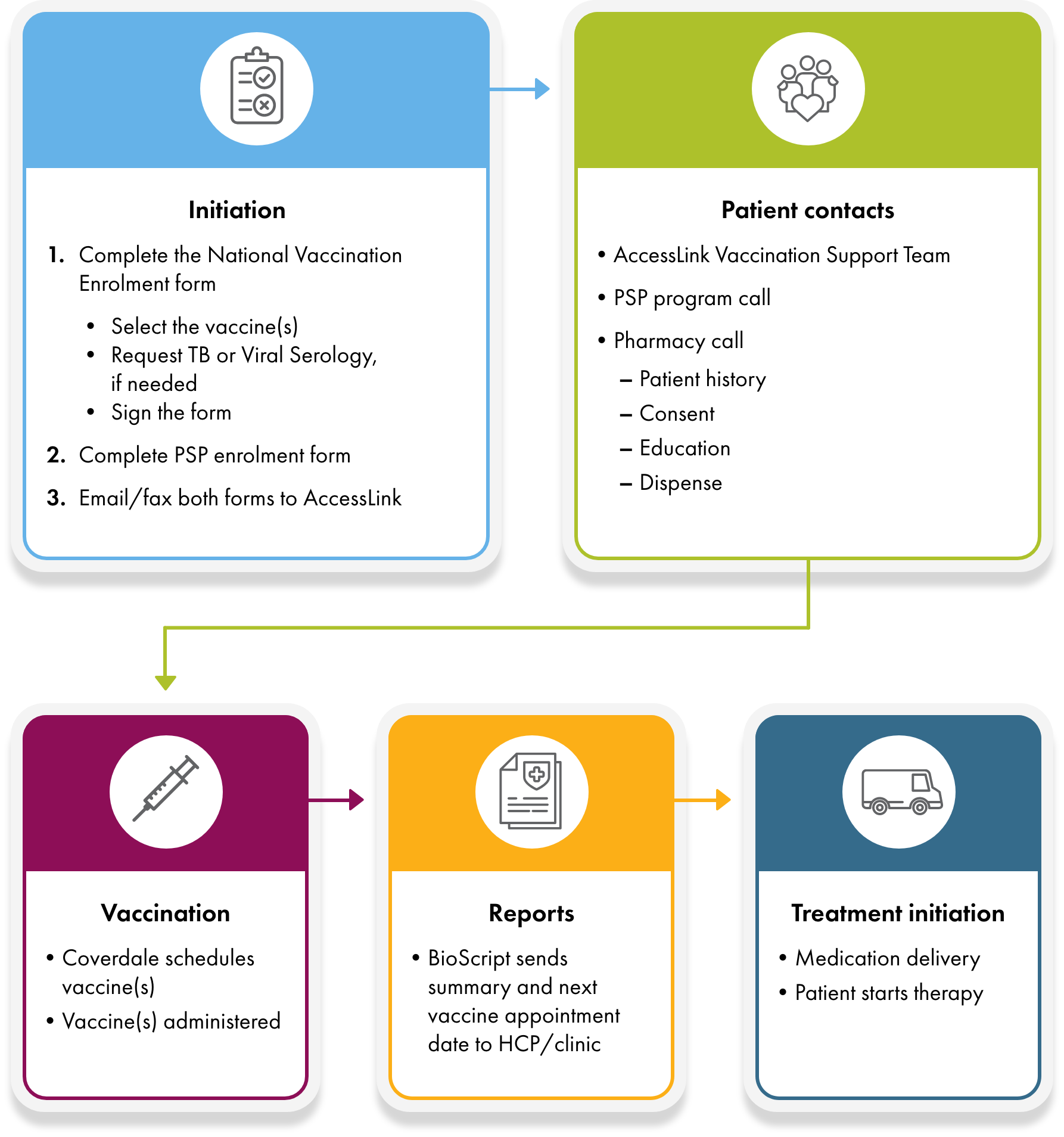
The program is designed to integrate into existing clinic workflows while reducing coordination challenges.
- A patient is identified as preparing to start immunosuppressive therapy.*,†
- Clinic submits vaccine and PSP enrollment forms to accesslink@bioscript.ca or by fax to 1-855-278-5182.
- BioScript Solutions contacts the patient to provide education and collect vaccination history.
- Tuberculosis (TB) and/or viral serology testing is coordinated, if needed.
- Vaccination appointments are scheduled through BioScript Solutions’ network.
- Vaccines are administered in accordance with NACI guidelines or as directed by the clinician.
- A post injection report(s) summary is provided back to the clinic to support timely treatment initiation.
Patient journey at a glance
Patient Journey - Vaccine & Treatment Flow (for HCPs)
National Vaccine Services Enrolment Form for Immune-Modulating Therapy
How to enrol in NIVP:
- Complete the vaccination enrolment form
- Select applicable vaccines based on clinical assessment
- Ensure the form is signed by the prescriber
- Submit to accesslink@bioscript.ca or by fax to 1-855-278-5182 BOTH:
Resources
NACI guidelines links for immunocompromised patients:
Immunization of immunocompromised persons: Canadian Immunization Guide - Canada.ca
Immunization of persons with chronic diseases: Canadian Immunization Guide - Canada.ca
Canadian Immunization Guide: Part 4. Immunizing agents - Canada.ca
*Immunosuppressive therapy includes long term steroid use, chemo radiation therapy, biologic or non-biologic therapies for rheumatologic conditions and IBD.
†The Canadian Immunization Guide recommends that individuals with incomplete or no immunization records be considered unimmunized and started on a catch-up schedule.

